In-beam ballistic control for hadrontherapy
The expertize and the past realizations of the LPC in instrumentation, particularly in electronics and in microelectronics for the high energy experiments at the CERN LHC, are used to develop new concepts in the context of medical imaging. The idea of PET (Positron Emission tomography) is proposed as a tool to control the treatment planning in the cancer therapy centers using hadrons, either protons or carbon ion beams.
The goal is to control the ballistic of the beam in the patient tissues by in-beam and real time detection of secondary particles, emitted during the irradiation of the patient. This method requires being able to detect with a huge efficiency, and with a minimum dead time, the secondary particles emitted when the beam hits the patient. These particles could be high energy photons (called prompt gammas), or charged particles like protons, or 511 keV gamma-ray photons from the annihilation of a positron. The positron emitters are themselves produced by the nuclear reactions between the incident ion and the nucleus of the target. This last case is the one of interest for PET-like detection.
We are very much involved in the development of a system to detect these 511 keV gammas issued from positron emitters, using a PET like technics. If the concept is strictly identical to those of a usual clinical PET (the conventional PET cameras are daily used for diagnosis, to search for cancer pathology), there are some constraints to take into account when trying to use it for the ballistic control in hadrontherapy:
- The activity to be detected is rather low. Typically, the activity induced by the nuclear reactions between the incident beam and the patient tissues is 3 orders of magnitude less than those injected in a conventional clinical PET scan.
- The isotopes created during the irradiation are short lived isotopes (few minutes to few 10 minutes life times) and these isotopes diffuse inside the patient tissues because of the human metabolism. To avoid both this activity wash-out and diffusion, it should be tried to measure this activity “on-line” during the patient irradiation.
- There is a large amount of background because of the other secondary particles, particularly the gamma prompts also produced by the nuclear reactions (deexcitation, evaporation…), and this background could seriously affect the 511 keV gamma measurements.
- The geometry of the detector is also specific, as it cannot be a complete ring like conventional clinical PET, as it should be adapted to simultaneous use with the beam irradiation of the patient.
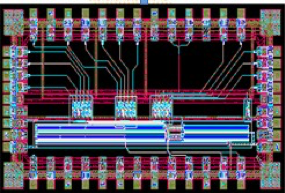
In the past years, the team focused on the utilization of the optimal filtering technics (PhD thesis of B Joly in 2010), already used for the ATLAS calorimeters. The amplitude and time of each signal is reconstructed from its sampling using a “running” ADC. The goal was to build readout electronics where one detection “pixel” (crystal) is associated to its preamplifier, its shaper and a sampling ADC. This choice lead to some development in electronics and microelectronics (development of an AMS 0.35 µm ASIC) , with the PhD theses of R Gaglione in 2006 and and of PE Vert in 2007 (see Fig. 1). All this work was made in collaboration with the electronics service of the IPN Lyon, and this initiated, together with other projects, the creation of the microelectronics pole named MICHRAU
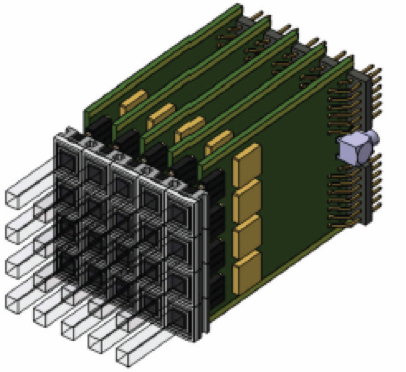
The years 2012-2015 have been dedicated to the construction of this detector, called the Large Area Pixelized Detector and to short periods of test, with parts of the full detector only, on various facilities: Centre de Protonthérapie d’Orsay, GANIL (Caen, France) and HIT (Heidelberg, Germany). These tests have been performed either with proton beams or carbon ion beams. The detector is made of 240 identical channels. Each channel is constituted of a LYSO crystal coupled to a photomultiplier tube (PMT). The PMT signal is sent to an Analog Sampling Module (ASM board) fully designed at LPC for this application.
After the construction, the first tests with the full detector have been performed at the Centre Jean Perrin at Clermont- Ferrand (CJP is both an hospital and a research unit from the INSERM (Institut National de la Santé et de la Recherche Médicale) and of the University of Auvergne. The experiment has been conducted with a phantom filled with a FDG (Fluoro-Désoxy-Glucose) liquid source, which has allowed to characterize the spatial resolution of the detector. On this particular subject, we have started collaboration with Z. El Bitar from IPHC (Strasbourg).

Since 2017, the LAPD is installed at the Institut Méditerranéen de ProtonThérapie, at Nice, on the Medicyc 65 MeV proton line, just before the treatment room. Tests have been performed to evaluate the capability of the LAPD to detect beam displacement, either due to target displacement or because of the presence of air cavities of various sizes in the target. Also, a huge effort is going on to upgrade the acquisition system from the VME based solution to a ATCA/µTCA based acquisition.

