Radiobiology-Biophysics
Research axes
Mitochondria, the cell powerhouse, are essential in cellular bioenergetics. They form either discrete units or dense networks throughout the cytoplasm and play key roles in vital processes by regulating energy production, ion fluxes, and essential metabolites.
Mitochondria possess their own DNA, maternally inherited and maintained by a dedicated set of proteins, different from the ones that maintain nuclear chromosomes. This mitochondrial DNA (mtDNA) is crucial for mitochondrial functions since it encodes most of the respiratory chain subunits.
Damage to mtDNA can have major pathological consequences in humans and has been linked to mitochondrial disorders, ageing, and cancer. These lesions can arise from replication defects or from reactive oxygen species, among other sources. However, many aspects of mtDNA maintenance and stability remain poorly understood.
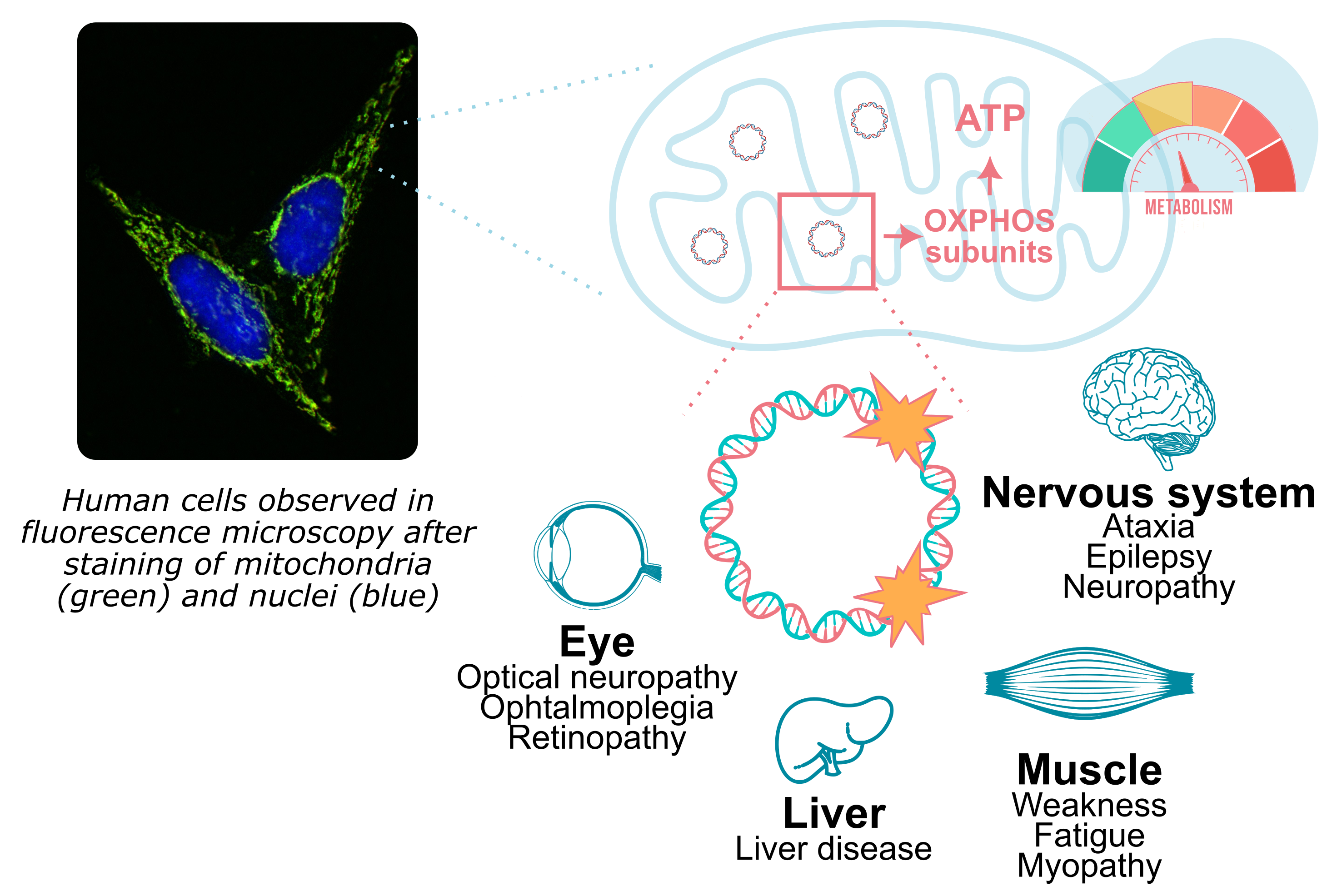
How do cells deal with mtDNA defect such as mutation in DNA maintenance proteins or direct DNA damage?
What is the mitochondrial response to X-ray exposure?
Deciphering mtDNA maintenance at the molecular level
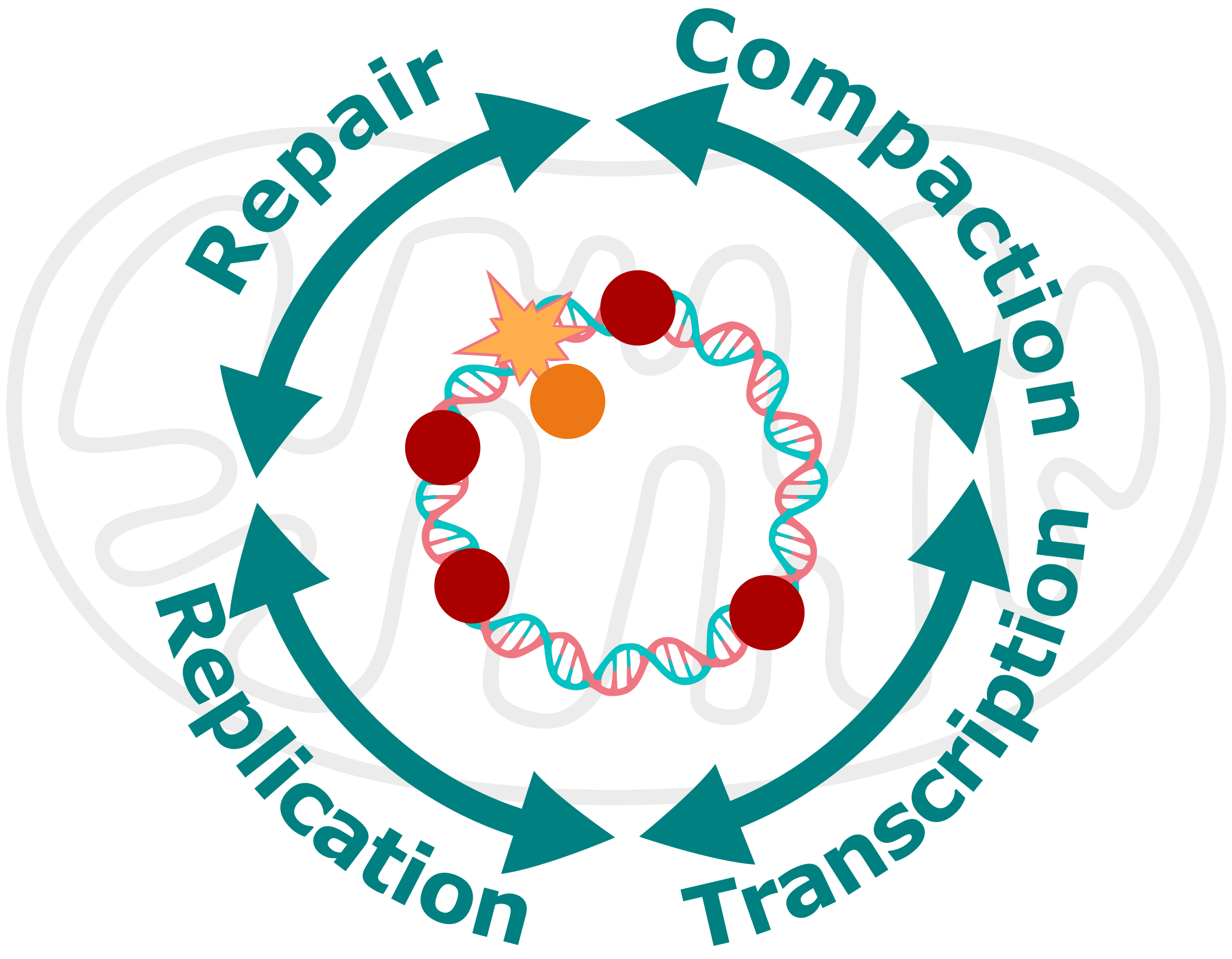
We investigate mtDNA maintenance mechanisms at the molecular level, to better understand how key processes such as replication, transcription, DNA damage repair and compaction are intertwined. Deciphering the interplay between these mechanisms and how they are affected in pathological conditions such as mutation of mtDNA maintenance proteins or overload of mtDNA damage is crucial to understand the basis of mtDNA disorders.
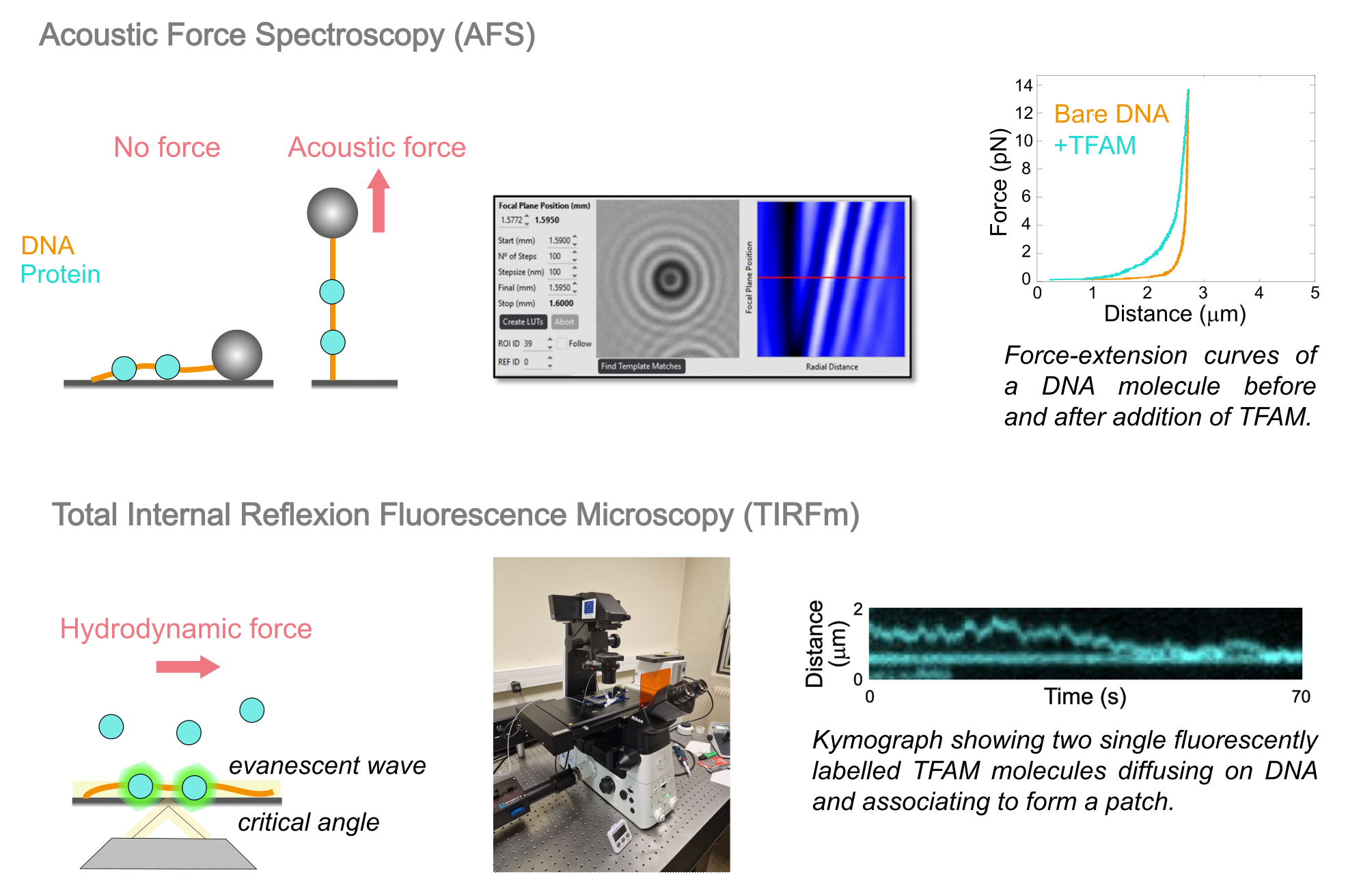
To this aim, we are mainly working in vitro with biophysics single-molecule approaches using microscopy, microfluidics, as well as bulk biochemistry techniques. The two state-of-the-art biophysics techniques we use in the lab are acoustic force spectroscopy (AFS) and total internal reflexion fluorescence microscopy (TIRFm). In AFS, a DNA molecule is attached between a polystyrene bead and a glass surface, and the bead can be manipulated with acoustic force, thus applying tension on the nucleic acid molecule. The analysis of DNA force-extension curves in absence or presence of recombinant mtDNA maintenance proteins, either wild-type or mutated, gives insight into their DNA binding affinity and mode. In TIRFm, the DNA molecules are attached to a glass surface and binding of recombinant mtDNA maintenance proteins labelled with a fluorophore can be directly visualized, in real-time.
Using these approaches, we have for example characterized the protein TFAM, a mitochondrial transcription factor that also plays a critical role in compacting mtDNA.
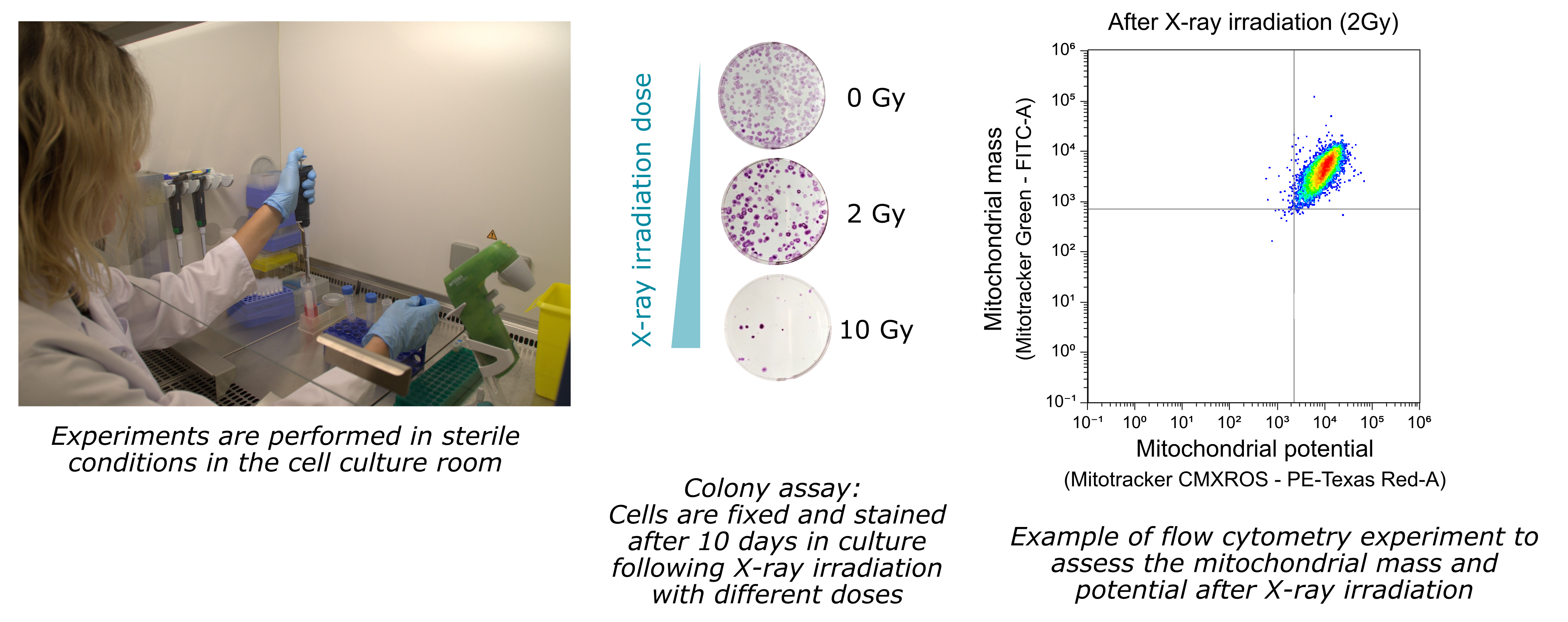
Investigating the effects of ionizing radiations at the cellular and mitochondrial levels
We investigate the mitochondrial behavior following X-ray exposure. Indeed, exposure of cells to ionizing radiation such as X-rays has many impacts on mitochondria, including, for example, the generation of damage to mitochondrial DNA, activation of energy production, and an increase in mitochondrial fission. We are therefore looking at the effects of X-ray exposure on mitochondrial parameters in human cell lines, either in basal state or after treatment with radiosensibilizing or radioprotective compounds targeting the mitochondria.
One goal of our studies is to tackle the current radioresistance challenge in cancer radiotherapies. Indeed, as mitochondrial metabolism is strongly involved in tumorigenesis and in response to ionising irradiations, we investigate the ability of selected compounds to target mitochondria in order to increase radiosensitization processes.
X-ray irradiation can be performed on site, on the PAVIRMA platform.
Collaborations and fundings
List of publications (last 5 years)
Lambert L, Moretton A, Farge G. Post-transcriptional modifications and regulation of mRNAs in human mitochondria. Biochimie. 2025 Nov;238(Pt A):9-18. doi: 10.1016/j.biochi.2025.06.015. Epub 2025 Jun 25. PMID: 40578749.
Martucci M, Moretton A, Tarrés-Solé A, Ropars V, Lambert L, Vernet P, Solà M, Falkenberg M, Farge G, van den Wildenberg S. The mutation R107Q alters mtSSB ssDNA compaction ability and binding dynamics. Nucleic Acids Res. 2024 Jun 10;52(10):5912-5927. doi: 10.1093/nar/gkae354. PMID: 38742632; PMCID: PMC11162770.
Debar L, Ishak L, Moretton A, Anoosheh S, Morel F, Jenninger L, Garreau-Balandier I, Vernet P, Hofer A, van den Wildenberg S, Farge G. NUDT6 and NUDT9, two mitochondrial members of the NUDIX family, have distinct hydrolysis activities. Mitochondrion. 2023 Jul;71:93-103. doi: 10.1016/j.mito.2023.06.003. Epub 2023 Jun 19. PMID: 37343711.
Martucci M, Debar L, van den Wildenberg S, Farge G. How to Quantify DNA Compaction by TFAM with Acoustic Force Spectroscopy and Total Internal Reflection Fluorescence Microscopy. Methods Mol Biol. 2023;2615:121-137. doi: 10.1007/978-1-0716-2922-2_10. PMID: 36807789.
Mehmedović M, Martucci M, Spåhr H, Ishak L, Mishra A, Sanchez-Sandoval ME, Pardo-Hernández C, Peter B, van den Wildenberg SM, Falkenberg M, Farge G. Disease causing mutation (P178L) in mitochondrial transcription factor A results in impaired mitochondrial transcription initiation. Biochim Biophys Acta Mol Basis Dis. 2022 Oct 1;1868(10):166467. doi: 10.1016/j.bbadis.2022.166467. Epub 2022 Jun 15. PMID: 35716868.

